If you will be participating in laboratory animal work at UConn Health in Farmington, please complete this form. Please fill out the form completely. Incomplete forms will be returned and training will not be scheduled.
uconn health
Research Excellence Program at UConn Health
dbGaP
- Request access to the controlled data sets from the appropriate dbGaP (Database of Genotypes and Phenotypes) Data Access Committee (DAC): https://dbgap.ncbi.nlm.nih.gov/aa/dbgap_request_process.pdf.
- Download and complete the model Data Use Certification (DUC) for the controlled data set of interest:
- Visit the dbGAP site at http://www.ncbi.nlm.nih.gov/gap.
- Search for the study from which data are requested. For example, “joint addiction, aging, mental health” results in number of studies.
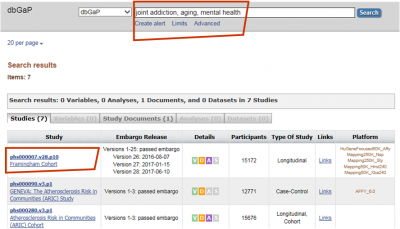
- Click on the linked study title of interest for instructions on how to download the model DUC and determine if IRB approval is required. Model DUC and IRB Requirements can be found under the “Study” tab in the “Authorized Access” section (as shown in screenshot).
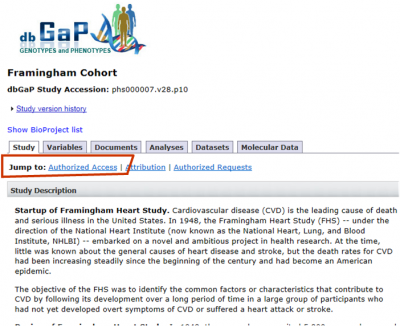
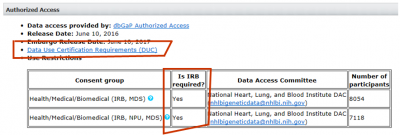
- If IRB approval is required, go to the section for “dbGaP Access Request” found at https://ovpr.uchc.edu//rcs/hspp/irb/irb-instructions-forms-and-samples/
download and complete the form “dbGaP Access Request Form for IRB Certification.”
- Complete the Data Security and Data Release Form (MS Word) (PDF).
- Email the completed Model Data Use Certification, IRB Approval for dbGaP Data Access (if required), and Data Security and Data Release Reporting form to the AVP for Research, Research IT Services – Dr. Khamis Abu-Hasaballah – at khamis@uconn.edu. Upon verification and approval of these documents, the AVP for Research will issue a certification letter signed by him, the Institutional Chief Security Officer, and the Institutional Signing Official (SPS Director).
Health Center Research Advisory Council (HCRAC) Contacts
Health Center Research Advisory Council
UConn Health
263 Farmington Avenue
Farmington, CT 06030-6403
For questions or comments about the HCRAC please contact one of the following individuals.
| Name | Title | Phone | |
|---|---|---|---|
| Christopher Heinen | Council Chair | (860) 679-8859 | cheinen@uchc.edu |
| Stephanie Holden | HCRAC Coordinator | (860) 679-2513 | sholden@uchc.edu |
Travel Grants for Postdoctoral Fellows
Application Guidelines and Instructions
This program has been established to support travel costs for postdoctoral fellows who present research results in-person at scientific meetings and symposia. The program will support costs of that travel up to a limit of $700 per fiscal year. Note, HCRAC travel funds cannot be combined with any other UConn travel support.
Awards are made on a first-come, first-served basis, until the funds for this program have been exhausted.
Eligibility
- Eligibility for this program is restricted to UConn Health postdoctoral fellows whose faculty preceptor for research is based at UConn Health and contributes their indirect costs to UConn Health.
- Applicants must be first author on a research abstract and present a corresponding poster or talk; the topic must be central to postdoctoral research performed at UConn Health.
- Postdocs may only receive one travel grant in a given year .
If there are any questions about this travel grant program, please contact Stephanie Holden at 860.679.2513 or sholden@uchc.edu.
InfoEd eRA Portal
 The UConn Health InfoEd is an enterprise-level, web-based application designed to manage all activities related to the management and execution of the research project life cycle – from cradle to grave. The system comprises a suite of modules, each designed to manage a specific aspect of the research project cycle. These include: proposal development and submission to the funding entity during the pre-award phase (Proposal Development – PD) ; proposal tracking and management in the post-award phase (Proposal Tracking – PT); human research protocol application management (Human Subjects Management – HSM); lab animal protocol application management (Lab Animals Management – LAM); environmental safety management (Environmental Safety Management – ESM); intellectual property management (Technology Transfer – TT); and clinical trial management, case report form (CRF) design, and scientific data collection and management (Clinical Trials Management – CTM). Modules currently installed at UConn Health include:
The UConn Health InfoEd is an enterprise-level, web-based application designed to manage all activities related to the management and execution of the research project life cycle – from cradle to grave. The system comprises a suite of modules, each designed to manage a specific aspect of the research project cycle. These include: proposal development and submission to the funding entity during the pre-award phase (Proposal Development – PD) ; proposal tracking and management in the post-award phase (Proposal Tracking – PT); human research protocol application management (Human Subjects Management – HSM); lab animal protocol application management (Lab Animals Management – LAM); environmental safety management (Environmental Safety Management – ESM); intellectual property management (Technology Transfer – TT); and clinical trial management, case report form (CRF) design, and scientific data collection and management (Clinical Trials Management – CTM). Modules currently installed at UConn Health include:
- Proposal Tracking (PT) – This module acts as a central clearinghouse of both pre- and post-award information. It provides a single reference point for tracking all details related to proposals including: budgets, subcontracts, approvals, technical reports, and all associated communications.
- Financial Conflict of Interests – This module provides researchers with the ability to complete and submit researcher and staff financial interest disclosures electronically. It allows Research Compliance Services staff to track and manage conflicts of interest, establish management plans, and fulfill our policy requirements. The module interfaces with proposals and human subjects. These materials include how-to instructions for UConn Health. Consult the FCOI website at UConn Storrs for the UConn Storrs and Regional Campuses FCOI how-to materials.
- Animal Facility Management / CCM Module – The InfoEd Animal Facility Management supports the animal care operations of the Center for Comparative Medicine (CCM) including animal ordering, husbandry, census, and billing of animal facility protocols.
- Lab Animal Use / IACUC Module – This module is designed to support the review and management of animal research protocols by the Institutional Animal Care and Use Committee (IACUC).
- Tech Transfer (TT) – This module was used to manage administrative data relating to intellectual property and tracks inventors, patents, key dates, contacts, agreements, documents and communications, and financial information tied to expenses and royalties. Now it contains legacy data on some University IP and was replaced by Wellspring Sophia.
- SPIN (Sponsored Programs Information Network) – SPIN is a searchable database that provides real-time access to current research funding opportunities. All data in SPIN is obtained directly from the sponsoring agencies themselves to ensure authenticity. To use SPIN, login to InfoEd and then choose Find Funding link at the top of the page.
- SMARTS (SPIN Matching And Research Transmittal Service) – SMARTS is an automated system that periodically queries the SPIN database and sends notifications to investigators regarding available funding opportunities in their field of study.
Sponsored Program Administration (SPA) Archive
- January 21, 2026 – Presentation Slides
- Agenda
- Pre-Award
- NIH: Biographical Sketch & Current and Pending Other Support Forms
- National Science Foundation: Policy Update
- Post-Award
- Guidelines for Faculty Maximum Effort on Sponsored Projects
- Training
- New LevelUp Modules
- NYC Research Administration Demonstration Series
- SPA Training Resources
- Pre-Award
- Agenda
- November 19, 2025 – Presentation Slides
- Agenda
- Research Security and Export Control – Lesley Salafia
- Pre-Award
- Proposal Submission
- Interim Guidance on Reopening of NIH Extramural Activities
- Post-Award
- Subawards Submission Tips and Tricks
- Student Fees
- Training
- LevelUp
- SPA Training Resources
- Agenda
- October 22, 2025 – Presentation Slides
- Agenda
- Financial Conflicts of Interest in Research – Meg Johnson
- Pre-Award
- Research Security training
- NIH: Other Support / Research Security Training
- Information and Compliance Form for Subrecipients
- Post-Award
- Outstanding Checks
- Travel Allocation
- SPA Training
- LevelUp updates
- SPA Training – New Resources
- Agenda
- September 17, 2025 – Presentation Slides
- Agenda
- IRB Updates – Shemetra Owens
- Pre-Award
- Research Security training
- NIH limiting submissions to 6 per calendar year effective 9/25/25
- Updated NIH NCE policy
- Post-Award
- Banner Budget – Travel
- Shipping / Express mail fees on awards
- Effort Reporting Audit – Early Outcomes
- SPA Training
- LevelUp updates
- New e-Learning modules now available in Saba
- SPA Training resources
- Training Resources job aid / website tour
- Agenda
- June 18, 2025 – Presentation Slides
- Agenda
- Introducing – Meg Johnson, Director of Conflicts of Interest and Research Integrity
- Research Finance
- Capital purchases – Capital Procurement Account Guidance
- Transfer vouchers
- Pre-Award
- Internal Forms: IPR and Subrecipient Forms Updated
- NIH updates
- NOFO Solicitation Requirements – Reminder
- PA-23-272—Parent F31 – Individual Predoctoral Fellowship update
- Post-Award
- Outstanding Checks
- AMS Rebudget process
- SPA Training
- 2025-2026 SPA Meetings – opinion poll
- Upcoming NCURA webinar – Research Administration in a Changing Federal Landscape
- Budgeting Basics e-learning course
- SPA Training resources
- Agenda
- May 21, 2025 – Presentation Slides
- Agenda
- Pre-Award
- Biographical Sketch: Adoption of the Common Form
- NSF: Implementation of Standard 15% IDC
- Foreign Subawards
- NIH No-Cost Extensions
- Research Security Training
- SPS Forms – Changes coming
- Post-Award
- Staffing Updates
- FY 2025 NRSA Stipend / Tuition
- Year End Reminders
- Executive Order – 14222
- Training
- LevelUP update
- New jobs aids – Budgeting Basics and Effort Reporting
- Training resources
- Pre-Award
- Agenda
- April 16, 2025 – Presentation Slides
- Agenda
- Pre-Award
- DHHS/NIH Update – Salary Cap
- Docusign – 5-Day Submission Policy
- 50K Program
- NIH Common Forms – Postponed
- Post-Award
- 2024 Single Audit
- Year End Reminders
- SPA Training Team
- LevelUp Update
- New EPAF update
- Training Resources
- Pre-Award
- Agenda
- March 19, 2025 – Presentation Slides
- Agenda
- Research Security Training Deadline – Lesley Salafia
- Pre-Award
- Federal Sponsor Updates
- NIH Common Forms (Required in May)
- NIH Fellowships – Forms-H
- Federal Sponsor Updates
- Post-Award
- DHHS Salary Cap Update
- Transfer Voucher Guide
- SPA Training Team
- Policy Manager Demo
- LevelUp Updates
- Training Resources
- Agenda
- January 15, 2025 – Presentation Slides
- Agenda
- Pre-Award
- NIH: FORMS-I
- Changes to NIH and AHRQ Fellowship Applications
- Pre-Award
- Agenda
-
-
- Post-Award
- FY 2025 Q2 Effort Reports
- Accounting for Costs – Uniform Guidance
- Accounting for Costs – Cost Accounting Standards (CAS)
- Accounting for Costs – HuskyBuy/Banner
- Training
- LevelUp updates
- New NoA Job aid
- Training resources
- Post-Award
-
- November 18, 2024 – Presentation Slides
- Agenda
- Financial Disclosures in Research – Gus Fernandez-Wolff
- Pre-Award
- Post-Award
- Food Related Expenses on Sponsored Awards
- Payroll & LDCA
- Staffing Update
- SPA Training
- SRA Modules & Training Initiative
- NIH Upcoming Events
- NSF Fall 2024 Virtual Grants Conference
- Agenda
- October 16, 2024 – Presentation Slides
- Agenda
- UCH Biosafety Program – Victoria Scranton, Biosafety Program Coordinator
- Pre-Award
- NIH – New Data Management and Sharing (DMS) Policy Questions in RPPR
- New DMS Section in Just-in-Time
- New ‘DMS Request’ in Prior Approval
- NIH Resources
- Acceptable/Unacceptable Signature Formats – Other Support pages
- Post-Award
- LDCA – By the Numbers
- AMS Rebudget
- Training
- Upcoming NIH Events
- New NCURA videos available on the website: The RPPR Matrix and Culture of Compliance
- Agenda
- September 18, 2024 – Presentation Slides
- June 26, 2024 – Presentation Slides
- Research Security & Undue Foreign Influence – NSPM-33 and CHIPS Act Requirements
-
- Agenda
- Export Control Overview – Mike Shelton
- Banner 9 upgrade
- Post-award
- FY 2024 NRSA Stipend / Tuition levels
- Year End Reminders
- Quick hits – Travel Authorizations
- Malign Foreign Talent Recruitment Program
- SPA Training – June meeting
- Agenda
-
- Agenda
- Foreign Travel / Export Control Updates – Mike Shelton and Bieu Tran
- Pre-award
- Federal Sponsor Updates - NSF
- Post-award
- 2023 Single Audit
- FY 2024 NRSA Stipends – NOT-OD-24-059
- Banner Upgrade
- P-Card Review
- Agenda
- March 20, 2024 – Presentation Slides
- Agenda
- Open Mike
- Financial Disclosure in Research- Gus Fernandez-Wolff
- Research Security – Mike Rock
- Pre-award
- Federal Sponsor Updates
- IPR Routing Form
- IPR Routing Form – PI Certification
- Agenda
- February 21, 2024 – Presentation Slides
- Agenda
- SPA Training announcements
- Pre-award
- Understanding NIH Application/Grant Numbers
- NIH Guidance on Marking Changes in Resubmission Applications
- NIH/DHHS Salary Limitation 2024
- Delegations in eRA Commons and RPPR Privileges
- Reports Due to Sponsor
- Post-award
- EPAFs, Tips, Tricks & Reminders
- Award Budget Reminder – Travel
- Agenda
- January 17, 2024 – Presentation Slides
- Agenda
- SPA Training announcements
- New On-Demand e-learning modules
- SPA Training Resources
- Pre-award
- Federal Sponsor Updates – NIH
- NIH Letters of Support
- OVPR Proposal Submission Policy
- Fellowship Applications – Submission Policy
- Post-award
- Cost Accounting Standards – 9905.502 – Banner Accounts
- AMS Rebudget Module – slides
- SPA Training announcements
- Agenda
- November 15, 2023 – Presentation Slides
- Agenda
- InfoEd version 13 (v13) Portal View
- Research Finance – F & A Base Year Follow-Up
- Tracking Students & Unpaid Occupants
- Preliminary Inventory Review
- Pre-award
- NIH Foreign Subrecipients – access to documents requirement
- Post-award
- Cost Transfer Best Practices
- SPA Training
- NCURA Region 1 – Spring meeting
- Resources
- December SPA
- Agenda
- October 18, 2023 – Presentation Slides
- Agenda
- Introducing the DMPTool – Adria Barbano, Academic IT Services
- Research Finance
- Reallocating Grant Fringe Budgets
- F & A Proposal- Base Year 2024
- Pre-award
- Changes to DSMP – Prior Approval
- Sample Routing Documents
- Proposal and Award Budget Templates
- Proposal Submission Policy
- New administrative staff – email lists
- Inform Your Faculty – reminder
- Cost Share Approval
- IBC and ICUC Grant Congruence Review Process Change
- Post-award
- F & A on Sponsored Projects
- SPA Training resources
- Agenda
- September 20, 2023 – Presentation Slides
- Agenda
- Pre-award
- DMPTool
- NIH RPPR – Section D
- NIH Data Management and Sharing Costs
- NIH – Hyperlinks Unallowable
- PI Eligibility – Fellowship Applications
- Proposal and Award Budget Templates
- Inform Your Faculty
- Research Fringe Benefits rates
- AMS Updates
- Post-award
- Reminders and New Processes for Better Documenting Cost Allocation
- Consulting Invoice Checklist
- Procurement/Contracts Information Session – 10/30, 1:00 p.m., Keller Auditorium
- SPA Training – Upcoming classes
- Pre-award
- Agenda
- May 17, 2023 – Presentation Slides
- Agenda
- Introduction – Bernard Cook, School of Medicine
- Updates to Funding Opportunity Terminology
- Policy – Qualifying Submissions
- Change to NIAMS Participation in NIH Parent R21 Announcements
- Year-End Reminders
- Training – class attendance
- Upcoming training classes
- Agenda
- April 19, 2023 – Presentation Slides
- Agenda
- eRA Commons News – Redesigned xTRAIN
- Compliance Reminder
- NIH Other Support Reminders
- IPR Compliance Crosswalk
- SPS Staffing Update
- 75521 Computing Devices – Guidance
- AMS Events
- SPA training – upcoming classes
- Agenda
- March 15, 2023 – Presentation Slides
- Agenda
- NIH Data Management and Sharing Policy – Reminders
- Proposal Tips and Tricks
- Indirect Costs on Cost-Shared Expenses
- Participant Support vs. Human Subjects Payments
- SPS Staffing Update
- Pre-Award Requests
- LDCA’s
- Account Code 75520
- SPA Training – upcoming classes
- Agenda
- February 22, 2023 – Presentation Slides
- Agenda
- Proposal Submission Policy
- Assist Applications
- eRA Commons Accounts – Personal Profile
- UCH as a subrecipient – reminders
- NSF Safe & Inclusive Workspaces – New Requirement
- NSF Audit Reminders
- FY 2023 NRSA Stipend/Tuition Levels
- Student Fees
- No Cost Extensions – Best Practices
- New Policies and where to find them
- CRA study group
- SPA Training Job Aids
- Agenda
- January 25, 2023 – Presentation Slides
- Agenda
- Confidentiality Agreements and Non-Disclosure Agreements
- SPS Staff Updates
- NIH Data Sharing and Management Plan Requirement
- NIH/DHHS Salary Limitation 2023
- NIH Project Roles
- Eligibility to Serve as PI
- Proposal Tips and Tricks – Cash vs. In-Kind Cost Share
- SPA Training Updates
- Agenda
- November 16, 2022 – Presentation Slides
- Agenda
- InfoEd updates
- OVPR Proposal Submission Policy
- Sample Routing Documents
- Proposal Routing Requirements Checklist
- ABP/Other Bonuses
- A/P Invoicing
- Subaward Requests in HuskyBuy
- AMS Events Module
- SPS Updates
- Overview of Agreements reviewed/negotiated by Contracts Group
- SPA Training Announcements and Updates
- Agenda
- October 19, 2022 – Presentation Slides
- Agenda
- IRB Presentation – Mayra Cagganello
- NASA Certification
- NIH Data Sharing & Management Plan
- Contract Submission Reminders
- Annual Data Report
- SPA Training – upcoming classes
- Agenda
- September 21, 2022 – Presentation Slides
- Agenda
- Environmental Health and Safety Updates
- Grants.gov Downtime
- NIH Genomic Data Sharing
- NCI Minimum Level of Effort
- NSF Fastlane
- Mobile Device Management
- Contract Services Group – updates
- SPA Training Updates
- Agenda
- June 22, 2022 – Presentation Slides
- Agenda
- Accounting for Costs – UG, CAS, HuskyBuy/Banner
- DHHS F & A Rate Agreement
- eRA Commons Enhancements
- Proposal Budget Template
- SPS Personnel Updates
- SPS Contact Assignments
- Greenphire update
- Agenda
- May 18, 2022 – Presentation Slides
- Agenda
- SPA Announcement
- June 2022 Updated RPPR Module and Instructions
- Internal Routing and Submission Deadlines
- Contracted Salary Increases – Budgets for Proposals
- Institutional Base Salary
- FY 2022 NRSA Stipend/Tuition Levels
- Agenda
- April 20, 2022 – Presentation Slides
- Agenda
- HuskyBuy Invoice Approval Automation
- Year end calendar
- New onboarding process for unpaid experiential
- AMS go-live update
- Personnel changes
- New fringe benefits rates to use for budgeting
- Proposal Routing – Documents and Timeline
- Flatten PDFs – JIT and RPPR
- Agenda
- March 16, 2022 – Presentation Slides
- Agenda
- 50K Program
- SPA Training updates – website updates and new Level One Basics class
- DocuSign updates
- DocuSign – resource discussion
- Other Support/In Kind Support FAQ’s
- Agenda
- February 16, 2022 – Presentation Slides
- Agenda
- PI Eligibility Fellowship applications
- New IPR Form
- DHHS/NIH Update – Salary Cap
- Subaward Invoice Review
- Mid Year Review
- Leadership update
- Agenda
- January 19, 2022 – Presentation Slides
- Agenda
- F&A Rate Agreement Renewal
- SPA Training
- NIH Era Commons Id For All Senior/Key Personnel
- NIH Submission Of Inclusion Enrollment Data
- NIH Annotated Forms G Set
- NIH Pre- And Post-Award Disclosures
- NIH Grants Policy Statement
- Uncashed Checks
- FDP Clearinghouse
- Agenda
- December 15, 2021 – Presentation Slides
- Agenda
- NIH New Requirements Updates
- NIH Prior Approval-Carryover-SPS Process
- Research Grant Reporting Request
- OVPR Routing Policy
- Agenda
- November 17, 2021 – Presentation Slides
- Agenda
- Training Update
- Open Mike
- Clinical Trials
- NIH Updates
- Agenda
- October 20, 2021 – Presentation Slides
- Agenda
- Research Development Services
- Banner 9 Upgrade
- Fall 2021 NIH Virtual Seminar on Program Funding and Grants Administration Registration
- eRA Commons Enhancement for Submit Reference Letter Screen
- Change to NIDCD Admin Reduction Guidance for Modular R01 Awards
- Proposal Dashboard Update
- Effort Certification After End Date
- Black Friday Update
- Agenda
- September 15, 2021 – Presentation Slides
- Agenda
- Fall 2021 NIH Virtual Seminar on Program Funding and Grants Administration Registration
- Unique Entity Identifier for Federal Sponsors
- NIH Updated Policy for Family Leave and Unpaid Leave for Extramural Loan Repayment Program
- NSF Website Enhancements
- Extending the Special Exception to the NIH/AHRQ/NIOSH Post Submission Material Policy During COVID-19
- NSF Pre-Award and Post Award Disclosures Related to the Bio-Sketch
- Scope of Work Change
- Approved Fringe Benefit Rates for 2022
- Agenda
- June 15, 2021 – Presentation Slides
- Agenda
- Institutional Base Pay
- NSF Publications Repository Changes
- New NIH Inbox for Biographical Sketch and Other Support Questions
- Revised NIH Grants Policy Statement
- eRA Commons Expanding Requirements for IDs
- What’s an Application Packet and When is it Needed
- Proposal Submission 9:00 am Due Date
- Agenda
- May 19, 2021 – Presentation Slides
- Agenda
- Biosafety Cabinet Certifications
- Year End Reminders
- Proposal Submission Policy Timeline and Reminders
- Fringe Benefit Rates
- NIH Biographical Sketch and Other Support
- Training Reminders
- Agenda
- April 21, 2021 – Presentation Slides
- Agenda
- Research Development Services Overview
- Salary Cap
- American Heart Association Carryover and No Cost Extension Updates and Reminders
- Publications and Printing Costs on Sponsored Awards
- IPR Routing Tips
- eRACommons Phasing Out Internet Explorer
- Redesigned RePORTTool
- eRACommons Login Tip
- NIH Biographical Sketch and Other Support
- Agenda
- March 17, 2021 – Presentation Slides
- Agenda
- NIH Update
- SPS Metrics YTD Comparison-Proposals/Awards
- Proposal Deadline Implementation
- Agenda
- February 17, 2021 – Presentation Slides
- Agenda
- IBC Discussion with Allison Pohl, Victoria Scranton, and Ron Wallace
- Student Fees
- PreAward Setup
- Updating PTR Before Hire
- NRSA
- Salary Cap
- Agenda
- December 16, 2020 – Presentation Slides
- Agenda
- Foreign Influence – Wesley Byerly
- OCTR Manager Change
- January Federal Costing Principles Module
- SPA Meeting Calendar Request
- ERA Commons Update
- Proposal Submission Policy
- Agenda
- November 18, 2020 – Presentation Slides
- Agenda
- MS SSRS Reports Update
- IPAS Discussion
- UConn Sponsored Program Metrics
- OVPR Proposal Submission Faculty Survey Results
- Proposal Submission Update
- Agenda
- October 21, 2020 – Presentation Slides
- Agenda
- Proposal Process – Under Development
- F&A on Projects of $50K or Less
- Sabbatical
- Principal Investigator Eligibility
- Use of Hypertext in NIH Grant Applications
- NIH Virtual Seminar
- OVPR Website
- Subaward Invoice Review
- Effort Reporting During Covid
- Agenda
- July 29, 2020 – Presentation Slides
- Agenda
- NIH updates
- NSF changes to award terms and conditions
- Spotlight on the new guidance for expenditures on sponsored awards
- NSF audit findings discussion
- Use of ASSIST
- gov resources
- Training videos
- Learn grants
- Visa costs
- Human subject participant payments
- Agenda
- June 24, 2020 – Presentation Slides
- Agenda
- OMB Expired Administrative Flexibilities
- HR Guidance Regarding Pay and Telecommuting
- Expenditures on Sponsored Awards Guidance
- Changes in HSS Post-Submission Updates with Forms-F
- PHS Human Subjects & Clinical Trials Information Form
- NIH, AHRQ, and NIOSH Updates
- Budgeting for Fringe Benefits on Sponsored Programs Updates
- OMB Expired Administrative Flexibilities
- Agenda
- May 26, 2020 – Presentation Slides
- Agenda
- Year-end Processing
- New Fringe Benefits Rates
- NIH Transition to Forms F
- COVID-related Information in RPPRs
- Requisitions needing SPS approval
- PPE Purchases
- Agenda
- April 16, 2020 – Presentation Slides
- Agenda
- COVID-19 Information
- Unique Entity Identifier
- NIH Updates
- NSF Updates
- FCOI Update
- Budget and Justification
- End of Grant
- Program Income
- Cost Transfers
- Subaward Invoice Review
- Fringe Rates
- Questions
- Agenda
- February 18, 2020 – Presentation Slides
- Agenda:
- Infographics Overview
- NIH Applications
- Change in Submission Deadlines and End of Recent Substantial Service Option
- Guidance on Video Submission
- SPS Routing Deadlines
- Certificate of Confidentiality
- Issuance of a Revised NSF Proposal and Award Policies and Procedures Guide
- DHHS Salary Cap
- NRSA Stipend/Tuition Levels
- Cost Transfer Update
- Question from the audience – Can you charge travel on a grant if not originally in the budget?
- Agenda:
- January 14, 2020 (there were no meetings in November or December, 2019) – Presentation Slides
- Agenda:
- May 2020 SPA Location Change
- NIH Public Access Policy
- IPAS Rebudget Requests
- NIH Multiple PI Certification
- NIH Human Subjects Decision Tool
- FDP Expanded Clearinghouse
- ORCiD
- NIH Submission Policies
- Agenda:
- October 15, 2019 – Presentation Slides
- Agenda
- NSF Audit
- New IPR Form
- IPR Highlight
- Effort on Federal Projects
- NIH Update
- SPS UConn Health Budget Templates
- Human Subjects Research
- New IPAS Form
- Change to Project Agreement Issuance
- Agenda
- September 17, 2019 – Presentation Slides
- Agenda
- Draft FDA Guidance with Dr. Wesley Byerly
- Fringe Benefits
- Use of Research Labs
- UConn Health OVPR Costs
- Training Updates
- Pre-Award Updates
- NIH Other Support
- NIH HFT Use
- ORCiD ID
- Sponsor Invoicing
- Cost Transfers
- Team Structure in SPS
- SPS Policy Updates
- Agenda
- May 28. 2019 – Presentation Slides and Compliance Slides
- Agenda
- CRA Study Group Update
- UCH Compliance Group (see compliance slides)
- Year End Processes
- 7630 Account Category
- Fringe Benefit Rates
- Salary Cap Updates
- Agenda
- April 30, 2019 – Presentation Slides
- Agenda
- Year End Dates
- Redesigned eRAWebsite
- NIH Funding Strategies
- Fringe Benefit Rates
- Salary Cap Updates
- Agenda
- March 26, 2019 – Presentation Slides
- Agenda
- Grant Writer Kit Bonin
- Training Update
- OIG Audit Overview
- NIH Updates
- Raises in July
- Agenda
- January 15, 2019 – Presentation Slides
- Agenda
- Training Update
- Export Control
- Fly America Act
- NIH Updates
- Agenda
- November 27, 2018 – Presentation Slides
- NIH Handouts
- Agenda
- New Website
- Training Update
- Review of FDP Agreement Request Form
- AMS Updates
- Transfer Vouchers
- Decoding NIH Grant Numbers (see NIH handouts)
- Sponsor Updates: NIH and NSF
- Agenda
- October 23, 2018 – Presentation Slides
- Agenda
- Training Update
- New Website
- Review of new ICF
- Procurement and Sole Sourcing
- Process for Contracts and Subawards
- Effort Reporting
- New F&A Agreement
- Sponsor Updates: NSF, NIH, and DOD
- Agenda
- May 2018
- March 12, 2018
- February 6, 2018
- November 8, 2017
- September 21, 2017
- May 24, 2017
- April 19, 2017
- March 8, 2017
- February 8, 2017
- January 11, 2017
- November 22, 2016
- October 19, 2016
- September 21, 2016
- June 15, 2016
- April 20, 2016
- March 2, 2016
- January 20, 2016
- November 18, 2015
- October 21, 2015
ResearchMatch
 ResearchMatch.org is a national online recruitment tool for health research, funded by the National Institutes of Health and maintained at Vanderbilt University. ResearchMatch connects health researchers with individuals interested in volunteering, through its secure online matching tool. UConn Health is part of the ResearchMatch Network. There is no cost to UConn Health researchers to use ResearchMatch. There are currently over 120,000 registered volunteers across the country. ResearchMatch is also now available in Spanish and simplified Chinese.
ResearchMatch.org is a national online recruitment tool for health research, funded by the National Institutes of Health and maintained at Vanderbilt University. ResearchMatch connects health researchers with individuals interested in volunteering, through its secure online matching tool. UConn Health is part of the ResearchMatch Network. There is no cost to UConn Health researchers to use ResearchMatch. There are currently over 120,000 registered volunteers across the country. ResearchMatch is also now available in Spanish and simplified Chinese.
To see if ResearchMatch might be a useful recruitment tool for your health-outcomes research study, use this link to register: https://www.researchmatch.org/researchers/. You do not need to be a Principal Investigator to register. The registration process takes less than 10 minutes. Once registered, you will be granted “feasibility access” to search volunteer aggregate data, including geographic and health demographics. Please note that you may not use ResearchMatch to recruit for a registry.
If you decide to use ResearchMatch to conduct participant recruitment, you will need IRB approval:
- The Vanderbilt IRB provides oversight for ResearchMatch as a recruitment tool and this has been documented within the ResearchMatch IRB Letter of Understanding (available upon request), but to use ResearchMatch as a recruitment tool for a specific study, you will first need to get UConn Health IRB approval.
- For the IRB submission, the following language may be used to describe ResearchMatch as a recruitment method: Potential volunteers will be contacted by ResearchMatch via an email contact message containing IRB-approved recruitment language for this study (not including direct study contact information such as email or phone number). Volunteers will then have the option of replying by clicking ‘yes’ or ‘no’ in the contact message. If a volunteer chooses to respond in the affirmative, they will authorize ResearchMatch to release their contact information to the PI (or ResearchMatch designee) who will be responsible for managing that information according to institutional guideline
- The contact message consists of the language to be used in the email sent by ResearchMatch on your behalf to potential participants. Please note that your contact message must not include your direct study contact information (email or phone), and must not exceed 2000 characters. If the study involves in-person participation, you may want to include the geographical location of the study site. If you intend to include healthy controls, specify that in your contact message, otherwise, a healthy control volunteer may decline participation. Please see contact message examples provided by ResearchMatch as well as this form that offers additional tips for creating an effective contact message.
- Once ResearchMatch is an IRB-approved recruitment method, you may then register as a researcher to request “recruitment access” in ResearchMatch. You will need to upload your IRB approval letter and your IRB-approved contact message. Recruitment access will give you the ability to send your contact message to potential participants that you select. If a potential participant agrees, you will then have access to his/her contact information in order to contact about possible study participation.
For questions about how to use ResearchMatch for your health research, please contact UConn/UConn Health ResearchMatch Liaison, Ellen Ciesielski, eciesielski@uchc.edu.
How to Register

2. On the PRS log-in page, in the Organization field, enter “UConnHealth” (no spaces) if your account was created under UConn Health or “UConn” if your account was created under UConn.
3. Click on the blue “Create New Record” button on the far right of the page. The system will walk you through the creation of the record. Data entered on previous pages will be retained so that you can return to complete the record at a later date.
The person who creates the record is the “Record Owner.” The Record Owner may be the Principal Investigator (PI) or someone designated by the PI to create/update the record on his/her behalf. The PI must perform the final review and approval of the record and each subsequent update in the PRS. The PI is responsible for ensuring that accurate information about the trial is entered and that updates to the record are completed in a timely manner (see table below for specific deadlines).
Complete the below fields as follows:
-
-
- Organization’s Unique Protocol ID: Enter the IRB number.
- Secondary IDs: Enter the grant number.
- Record Verification Date: Enter the month and year in which you are completing the record. This field will need to be updated each time you update the record after it is registered.
- Primary Completion Date: Enter the anticipated final data collection date, specifically regarding the Primary Outcome Measure.
- Responsible Party: Select the Principal Investigator from the drop-down menu.
- Collaborators: Add any other organizations providing support, including funding, design, implementation, data analysis and reporting.
-
For studies reviewed by UConn Health:
-
-
- Board Name: UConn Health IRB
- Board Affiliation: UConn Health
- Board Contact phone and e-mail: 860.679.8729; irb@uchc.edu
- Board Contact Address: UConn Health IRB, 263 Farmington Avenue, Farmington, CT 06030-1511
-
For studies reviewed by UConn Storrs and Regional Campuses:
-
-
- Board Name: UConn IRB
- Board Affiliation: UConn
- Board Contact phone and e-mail: 860.486.0986; irb@uconn.edu
- Board Contact Address: UConn IRB, 438 Whitney Road Extension, Unit 1246, Storrs, CT 06269
-
The Final Rule dictates that ClinicalTrials.gov record fields are updated on the below schedule:
| Data Field | Deadline for Updating (i.e., not later than the specified date) |
|---|---|
| Study Start Date | 30 calendar days after the first subject is enrolled (if the first human subject was not enrolled at the time of registration). |
| Intervention Name(s) | 30 calendar days after a nonproprietary name is established. |
| Availability of Expanded Access | 30 calendar days after expanded access becomes available (if available after registration); and 30 calendar days after an NCT number is assigned to a newly created expanded access record. |
| Expanded Access Status | 30 calendar days after a change in the availability of expanded access. |
| Expanded Access Type | 30 calendar days after a change in the type(s) of available expanded access. |
| Overall Recruitment Status | 30 calendar days after a change in overall recruitment status. If Overall Recruitment Status is changed to “suspended,” “terminated,” or “withdrawn,” the Why Study Stopped data element must be submitted at the time the update is made. |
| Individual Site Status | 30 calendar days after a change in status of any individual site. |
| Human Subjects Protection Review Board Status | 30 calendar days after a change in status. |
| Primary Completion Date | 30 calendar days after the clinical trial reaches its actual primary completion date. |
| Enrollment | At the time the primary completion date is changed to “actual,” the actual number of participants enrolled must be submitted. |
| Study Completion Date | 30 calendar days after the clinical trial reaches its actual study completion date. |
| Responsible Party, by Official Title | 30 calendar days after a change in the responsible party or the official title of the responsible party. |
| Responsible Party Contact Information | 30 calendar days after a change in the responsible party or the contact information for the responsible party. |
| Device Product Not Approved or Cleared by U.S. FDA | 15 calendar days after a change in approval or clearance status has occurred. |
| Device Product Not Approved or Cleared by U.S. FDA | 15 calendar days after a change in approval or clearance status has occurred. |
| Record Verification Date | Any time the responsible party reviews the complete set of submitted clinical trial information for accuracy and not less than every 12 months, even if no other updated information is submitted at that time. |