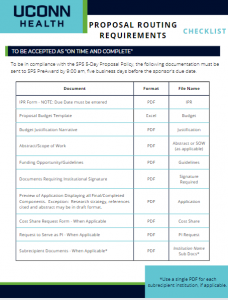
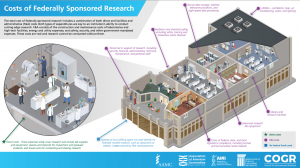
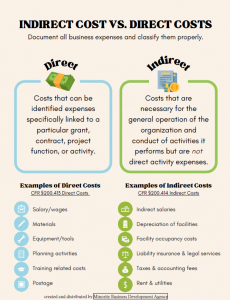
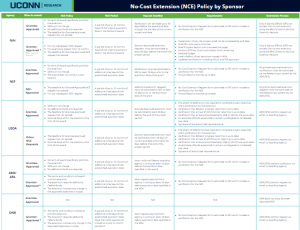
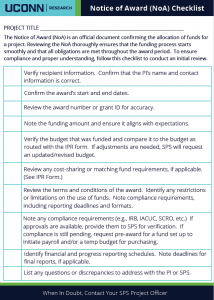
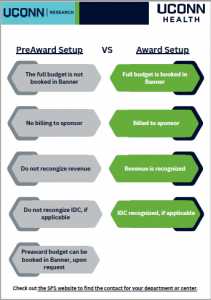
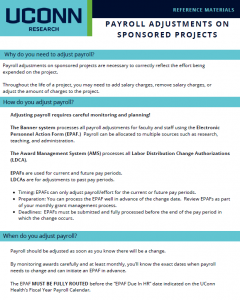
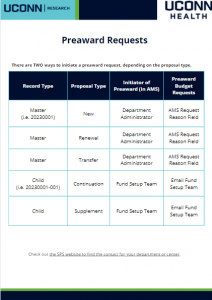
The Sponsored Programs Administrators (SPA) Meeting is held monthly during the academic year to provide information to department administrators. SPA meetings are typically held the 3rd Wednesday of the month at 2:00 p.m. Please send any suggestions or comments to SPA.training@uconn.edu.
For a full list of all SPA Training Team training resources, check out the extensive list on our job aids webpage.
Next Meeting – February 18, 2026, 2:00 p.m.
Upcoming Meetings
March 18, 2026
April 15, 2026
May 20, 2026
June 17, 2026
For information from previous meetings, visit the SPA Meeting Archive.
Sponsored Program Services (SPS) is developing a comprehensive research administration education program. The program will include in-person training sessions, video tutorials, reference guides, and other workshops and events meant to provide support and professional development for research administrators, faculty, and students involved in research.
As the program develops, opportunities will be listed on the links available on the sidebar. Currently there is information in SPA Training Opportunities.
For a full list of all SPA Training Team training resources, check out the extensive list on our job aids webpage.
Please send any suggestions or comments to SPA.training@uconn.edu.
Export control laws are federal regulations that govern how certain information, technologies, and commodities can be transmitted overseas or to a foreign national on U.S. soil. The scope of the regulations is broad: they cover exports in virtually all fields of science, engineering, and technology and apply to research activities regardless of the source of funding. Failure to comply with these laws can have serious consequences, both for the institution and for the individual researcher. Potential penalties include fines and possibly imprisonment. It is thus critical for UConn Health researchers to understand their obligations under these regulations and to work with Research Compliance Services to ensure that the University is in compliance.
Please visit this section in the Research Integrity & Regulatory Affairs area of the website for more information.